DRBC agar: Principle and preparation
Introduction
Dichloran rose-bengal chloramphenicol (DRBC) medium was first formulated by A. Douglas King, Jr. and colleagues in 1979. DRBC medium is an improvement over rose-bengal chloramphenicol (RBC) medium that facilitates the enumeration of certain fungal species that cannot be accomplished using RBC medium.
Douglas King, Jr. and colleagues screened thirty-one compounds known for their fungistatic or fungicidal effects with the aim of identifying those that could control the overgrowth of fungi on culture media, thus allowing for better enumeration. Among those thirty-one compounds, they identified two, dichloran and pentachloronitrobenzene, that had the desired effect of restricting the overgrowth of fungi without affecting the germination of spores. However, they proceeded to further evaluate only dichloran as pentachloronitrobenzene is a suspected carcinogen.
The researchers tested compositions of dichloran and rose-bengal with varying concentrations to identify one which allowed enumeration without disturbing the colony-forming unit (CFU) count. They found that dichloran concentration inversely affects the fungal colony diameter, making it possible to alter its concentration in the medium based on the growth characteristics of the fungal species under study.
Principle
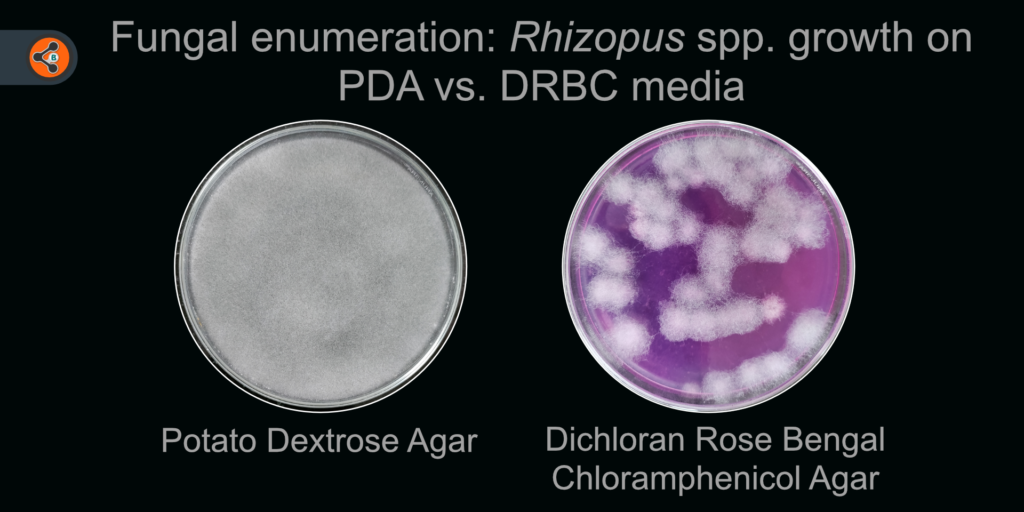
- Dichloran & Rose-Bengal: Restricts the overgrowth of spreading mold. In general, CFU size is inversely proportional to the concentration of dichloran and rose-bengal. The following table gives us a glimpse into the relation between colony size and dichloran concentration.
| Dichloran concentration (µg/ml) | Reduction of colony diameter in comparison to control (in %) |
|---|---|
| 1 | 13 |
| 5 | 41 |
| 10 | 66 |
| 25 | 74 |
| 50 | 78 |
| 100 | 81 |
Table 1. Average colony diameter of molds at different dichloran concentrations on maltose agar (MA) medium.
Although dichloran reduces mold colony diameter for most species of fungi, it is less effective with yeast where the change in colony diameter is less than 10% even at high concentrations.
Needless to say, the size and growth rate of the fungal colony on DRBC agar depends on the species, as shown in Table 2.
| Species | Growth rate (mm per day at 25°C) |
|---|---|
| Rhizopus stolonifer | 8.1 |
| Aspergillus flavus | 4.5 |
| Penicillium cyclopium | 3.5 |
Table 2. Growth rate of fungi varies on DRBC agar.
- Chloramphenicol: It is a broad-spectrum antibiotic that inhibits bacterial protein synthesis by binding to their ribosomes.
- Dipotassium Phosphate: Buffering agent.
- Magnesium sulfate: Trace elements.
- Peptone: Source of carbon and nitrogen.
- Dextrose: Energy source.
Applications
- For enumeration of fungal species found in food samples.
- Selective isolation of yeasts and molds from food and soil samples.
Advantages of DRBC medium
- It prevents bacterial growth to some extent due to low pH and chloramphenicol.
- Some fungal and bacterial species take up rose-bengal (bacterial colonies can be observed in plates lacking chloramphenicol). This makes colony counting easier.
- It is superior media than RBC as most fungal species give more colony count comparatively.
Disadvantages
- At higher concentrations, dichloran affects conidiogenesis in Niger spp. At a concentration of 5 µg/ml and above, conidiogenesis is completely inhibited. However, at 2.5 µg/ml, conidiogenesis is largely unaffected.
- Colony morphology is affected in the case of molds at a dichloran concentration of 5µg/ml and above, which makes identification of species difficult. In such cases, subculturing is recommended.
Composition
*Chloramphenicol is optional and is used in order to prevent bacterial contamination.
| Component | For 1L media (in grams) |
|---|---|
| Peptone | 5g |
| Dextrose (Glucose) | 10g |
| Potassium dihydrogen phosphate | 1g |
| Magnesium sulphate | 0.5g or 500mg |
| Rose Bengal | 0.025g or 25mg |
| Chloramphenicol | 0.100g or 100mg |
| Dichloran | 0.002g or 2mg |
| Agar | 15g |
Table 3. Composition of DRBC agar.
pH
Although the medium can be used at pH 7.2, a pH ranging between 5.40 − 5.80 (average 5.6) is recommended to inhibit bacterial growth. A higher pH, like 7.2, not only increases the risk of bacterial contamination, but also leads to faster growth and larger colonies of fungi.
Preparation
- Add required quantities of all the components except for agar and chloramphenicol in 800 mL of distilled water.
- Adjust the pH and make up the volume to 1L.
- Aliquot the solution if necessary.
- Add agar.
- Sterilize by autoclaving at 121°C for 20-30 minutes.
- Add chloramphenicol when the medium has sufficiently cooled down.
- Pour plates. Seal with parafilm.
Storage
- Wrap bottles containing DRBC medium with aluminum foil to avoid exposure to light.
- DRBC medium can be stored at 4°C for a maximum of 4 weeks.
- Use freshly poured plates within 2 weeks.
- If plates are made by reheating stored DRBC agar, use them within a week.
Points to be noted
- Add agar after adjusting the pH of the medium to avoid hydrolysis of agar.
- Protect the liquid medium and plates from light. Rose-bengal is light-sensitive, and its photodegradation product is toxic to fungi.
- Do not use the medium which is past its recommended storage time as the colony count can be affected.
Reference
- Dichloran-Rose Bengal Medium for Enumeration and Isolation of Molds from Foods.
- Quality control of two rose bengal and modified DRBC and DG18 media.
- Himedia.
- Inhibition of Growth of Fungi on Rose Bengal Media by Light.
- Dichloran as an Inhibitor of Mold Spreading in Fungal Plating Media: Effects on Colony Diameter and Enumeration.