Sabouraud dextrose agar (SDA)
Introduction
The Sabouraud Dextrose Agar (SDA) or Sabouraud agar medium was first formulated by a French Physician Dr Raymond Jacques Adrien Sabouraud (pronounced sah-bū-rō’), in 1892 while investigating fungi which cause skin lesions. SDA is a selective media for fungal culture and primarily used for the isolation of Dermatophytes, yeasts and various other pathogenic and non-pathogenic fungi. Dermatophytes are a group of closely related fungi that can invade keratinized tissue such as skin, nails and hair of humans and other animals. SDA media also supports the growth of filamentous bacteria such as Nocardia spp.
This media is widely used for research and clinical studies. The conventional composition of the media does not include antibiotics but relied on low pH (5.6) for the inhibition of bacterial growth.
However, for specific clinical uses, the traditional SDA media is often modified by adding various antibiotics to augment the antibacterial effect.
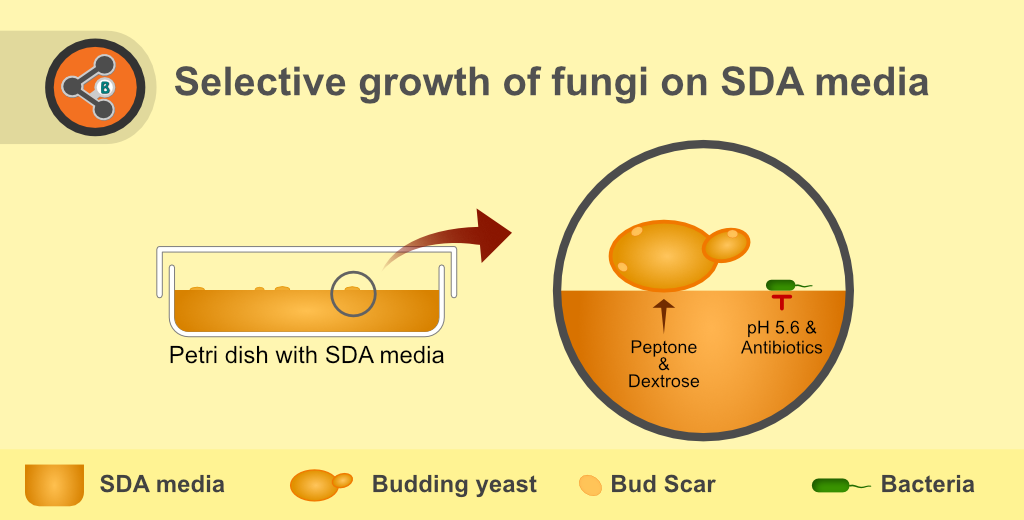
Principle
The three essential components of the SDA medium, which confer the growth of fungi are peptone, dextrose (glucose) and pH. The mycological peptone (a mixture of animal and plant peptones) provides a nourishing source of amino acids and nitrogenous compounds while dextrose acts as a source of carbon and energy. Both of these components help the rapid growth of the fungi. Whereas, agar (solidifying agent) aids to get morphological details of the colony. The components mentioned above can be modified by adding the various substances as per the requirements of the fungi.
The final pH of the medium is adjusted to 5.4 – 5.8, preferably the 5.6 at 25 °C. pH plays an essential role in the selective growth of fungi. pH 5.6 is preferred for composing culture media in the pre-antibiotic era to minimise bacterial contamination. At pH ~5.6, most types of bacterial growth is inhibited. However, bacteria that can thrive in an acidic environment manage to grow on this media. Hence, besides the pH, broad-spectrum antibiotics (e.g. chloramphenicol, tetracycline and Gentamycin) are used to augment the antibacterial effect.
percentage of glucose used in the media also helps in minimising bacterial contamination. A high percentage of glucose leads to the vigorous growth of bacteria, which leads to higher fermentation and acid production, later inhibiting bacterial growth.
Figure 1.Illustration depicting the principle of Sabouraud Dextrose Agar (SDA) or Sabouraud agar medium.
Applications
SDA is primarily used for selective isolation of fungi including dermatophytes, yeasts and aciduric bacteria. The uses include but not limited to
- Mycological analysis of food, cosmetics, personnel hygiene, surfaces and air.
- Clinical specimens for fungal contamination.
- It is also used for the recovery and total counting of yeasts and moulds in environmental monitoring.
Composition
| Reagent | For 100 mL | For 500 mL | For 1 L |
|---|---|---|---|
| Dextrose (glucose) | 4 g | 20 g | 40 g |
| peptone (or mycological peptone) | 1 g | 5 g | 10 g |
| Agar | 1.5 g | 7.5 g | 15 g |
Table 1. Composition of Sabouraud dextrose agar.
Emmons version of Sabouraud Dextrose Agar and the broth has only 20.0 gm of dextrose and 10.0 gm of neopeptone per one litre of culture media and final pH of 6.9 +/- 0.2 at 25ºC.
There are other variations for the SDA media which differ in quantity of components, differs in composition and/or pH. These variations are to give an advantage for the growth of certain species or to obtain differential growth.
Sabouraud dextrose broth
SDB has the same formulation as above, without agar added.
pH
Traditionally, the pH of SDA media is slightly acidic (pH 5.6 +/- 0.2 at 25ºC.). pH to be adjusted with 1N NaOH and 1N HCl.
There are other versions of SDA media with variations in pH.
Use of antibiotics
Antibiotics like chloramphenicol (50.0mg/L), gentamicin (5.0mg/L), and tetracycline (10.0mg/L) can be added for successful isolation of fungi and yeasts while inhibiting bacterial overgrowth. Use of antibiotics is helpful when inoculum is overloaded with bacteria.
Note that the use of antibiotics may affect the growth and morphology of some fungal species. It is impotent to investigate the compatibility of the antibiotic used and the fungal species intended to isolate or detect. Here we are providing a brief list of antibiotics and fungal species that get affected with the antibiotic.
Cycloheximide – Cryptococcus neoformans, Aspergillus fumigatus and Allescheria boydii
Penicillin and streptomycin – Actinomyces bovis and Nocardia asteroids
for more detailed about use of antibiotics used in SDA media, please refer to Sabouraud agar.
Preparation
- Weigh the ingredients separately with respect to the volume of the media. (Here, we are considering 1L of the media).
- Suspend the ingredients (except agar) in a glass beaker containing about 900mL of dd H2O.
- Dissolve the components in the beaker using a magnetic stirrer. (Heat may be applied to dissolve the medium completely).
- Adjust the pH of the medium to 5.6 or the desired value.
- Adjust the broth to a final volume of 1L using ddH2O.
- Transfer the broth to conical flask or aliquot into smaller volumes.
- Now add agar accordingly with respect to the volume of the media (i.e., 15 gms agar for 1L of the media).
- Close the mouth of the flask with a cotton plug. Seal it further with paper and rubber band.
- Autoclave for 20 min at 15 psi (1.05kg/cm2) on liquid cycle.
- However, if antibiotics are to be included, their stock solutions should be filter sterilized before addition to the media. These antibiotics must be added after the media is cooled to about 45-50ºC.
- Mix well and pour into sterile Petri plates or tubes for slants.
Alternatively, commercially available SDA media powders can be used. Weigh the mixture of content as prescribed by the manufacturer.
Method of inoculation and incubation
Method of inoculation and incubation may vary based on the source of inoculum. For example
- Streaking – isolation
- Monitoring air quality – the lid of Petri plates oped for a specified time in a specific place
- Surfaces – swabs sample
- Personal hygiene monitoring – fingerprints
In general, the samples are inoculated in duplicates.
- One set is to be incubated in 22-25ºC and other is in 30-37ºC. This allows the growth of fungi which require different temperatures and allows dimorphic fungi identification.
- yeasts are incubated at 28-30ºC
- Keep the lid/cap loose to allow the exchange of gases and moisture. most of the dermatophytes and molds are obligate aerobes.
- Examine plates every 2-4 days. Some of the dermatophytes or dimorphic fungi (Histoplasma capsulatum) require 2-4 weeks of
Interpretation of results
Results are interpreted based on colony macroscopic details such as morphology, texture and colour. In addition to this, the time required to growth, source of inoculum, media composition and use antibiotics helps narrow down the results. It is important to observe the morphology of the colony from the bottom of the Petri plate. Having positive controls (plates with known inoculum) would certainly come handy.
Limitations
- Some strains may be encountered that grow poorly or fail to grow on this medium.
- Apart from bacteria, certain pathogenic fungi may also be inhibited by the antimicrobial agents added into the medium.
- For identification purpose, organisms must be in pure culture.
- Morphological, biochemical, and serological tests should be performed for final identification.
- Use of protective cabinets is recommended while handling the samples with pathogenic fungi to avoid the spread of spores.
Storage
Store the broth and plates at 4ºC until they are utilized, regardless of whether they contain antibiotics.
Points to be noted
- Avoid overheating a medium with an acidic pH, as it may lead to a soft medium due to hydrolysis of agar.
- Peptone is hygroscopic. Store it in an air-tight container.
- Variation in the reagents (lot to lot and vender to vender) may influence colony morphology and rate of growth.