Animal life and survival primarily depend on their ability to respond to the environment around them. These controlled responses are a result of highly specific functions of the nervous system present in an animal. Calcium (Ca2+) is one of the primary signalling molecules which governs the specificity of neuronal functions via controlling at individual neuron (the functional units of the nervous system).
There are two primary classes of proteins called neuronal calcium sensors (NCS) and neuronal calcium-binding proteins (nCaBPs) which play a primary role in neuronal signaling. These proteins precisely sense the change in Ca2+ concentration in neurons and controls the downstream signaling. Here we worked on a vertebrate-specific protein called Caldendrin (CDD), a nCaBP present in dendritic spines, involved in neurotransmission and memory. The mice devoid of CDD exhibit deficits in spatial learning and memory state the importance of the CDD.
Although the CDD was convinced to be important Ca2+ sensor, the mechanism of the calcium-binding was poorly understood. CDD does not show much secondary and tertiary structural changes as it binds to calcium, unlike other calcium sensors, makes it further unique and complex. It is essential to understand the structure of CDD before diving into the mechanism.
CDD has a unique bipartite structure (Fig 1). The N terminal domain is a proline-rich disordered domain, contains seven phosphorylation sites and several PxxP motifs. The C-terminal domain contains 4 EF-hands (Ca2+ -binding motifs). Out of the 4 EF-hands, only 3 and 4 (purple) binds Ca2+. The EF1 hand (yellow) has a higher affinity for Mg2+ and its ability to bind Ca2+ is not consistent in literature, whereas EF2 is non-functional (grey).
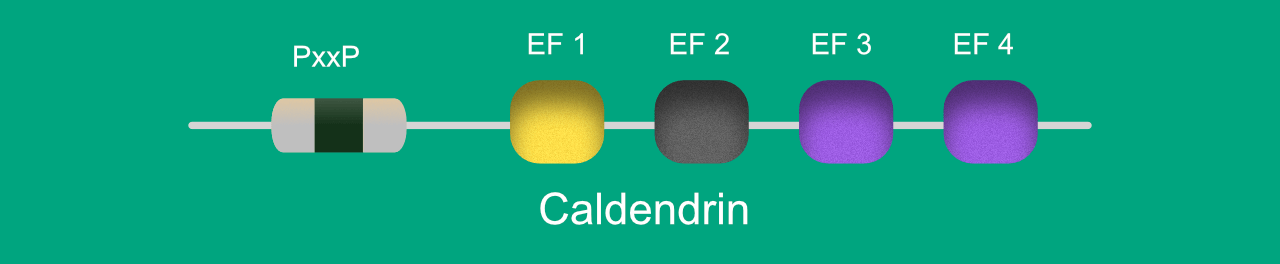 Fig 1. Illustration depicting the structure of caldendrin.
Fig 1. Illustration depicting the structure of caldendrin.
To investigate the structural role of individual EF-hands in the function of CDD, we introduced Tryptophan (Trp, a natural fluorescent amino acid) at each EF-hand (EFs) explicitly. This method is called “EF-hand scanning Trp mutagenesis”. In brief, “EF-hand scanning Trp mutagenesis” involves the replacement of specific amino acids one at a time with Tryptophan to study the microenvironmental changes with fluorescence spectroscopy. In essence, the data generated will let us know the ligand binding properties and stability of each EF-hand under experimental conditions.
The fluorescence and unfolding studies of individual Trp reporter proteins led to the finding of unique inter-motif Communication, i.e. binding of Ca2+ at EF3 lead to destabilization of EF4. After the complete saturation of EF3, the EF4 regains its stability. The whole process is mentioned stepwise below.
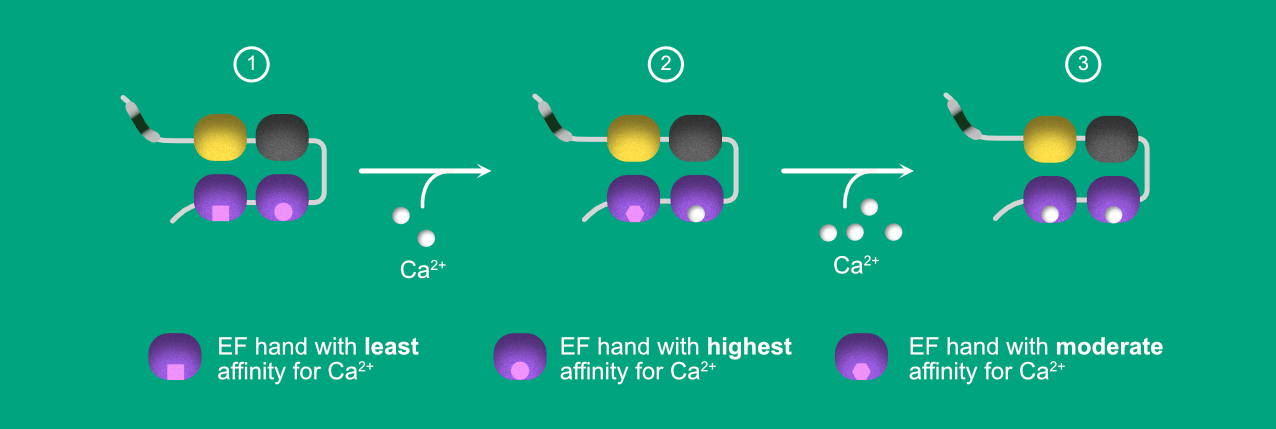 Figure 2. Illustration depicting calcium binding and inter domain communication between EF3 and EF4.
Figure 2. Illustration depicting calcium binding and inter domain communication between EF3 and EF4.
- STEP1: In APO (no Ca2+) state, EF3-hand has a high affinity for the Calcium ions. Hence it gets occupied even under low calcium concentrations. Fig 2 shows the same by representing the Calcium ion binding site as spherical, whereas the EF4-hand has low affinity for Ca2+ compared to EF3, represented in Square shaped binding pocket.
- STEP2: When the protein is exposed to the low Ca2+ concentrations, EF3 becomes saturated. The ligand binding to EF3 triggers the conformational changes in the EF4, resulting in increased affinity for the EF4 to Ca2+. However, the affinity is still lower than the EF3. In other words, to saturate EF4-hand, we still require higher concentrations of Ca2+ compared to EF3.
- In STEP 3, after providing the high Ca2+ concentration, EF4- hand saturates with Ca2+.
Further, we have conducted experiments with wildtype CDD (unmodified primary sequence) protein to rule out the possibility of disturbance caused by Tryptophan. The unfolding of wild-type (WT) CDD, was monitored by external fluorophore 8-Anilinonaphthalene-1-sulfonic acid (ANS). ANS when tagged to a protein, reports changes in the microenvironment (relative hydrophobicity) by changing its fluorescence properties.
The data obtained with ANS experiments show the unstable intermediate. At the same time, the CDD transit from apo (without Ca2+) to halo (with Ca2+) states at different Ca2+ concentrations before it reaches the Ca2+ saturated state. These results fortify the findings from “EF-hand scanning Trp mutagenesis”.
When EF3 is mutated to abolish its Ca2+ binding ability, the inter-motif communication does not occur. From this study, it is evident that in CDD, EF3 play a significant role in its unique inter-motif communication, which believed to play a role in functional aspects of the protein with respect to neuronal signaling.
Primary reference:
Inter-motif communication induces hierarchical Ca2+-filling of Caldendrin
[simple-author-box]

3 comments
Jitendra
Good job in Ca2+ signalling
parthasaradhi
Glad you liked it Jitendra 🙂
Jitendra
Good job!