Optineurin is a protein expressed in most of the organs like brain, eyes, spinal cord, muscles, liver and heart. It has functions in several cellular processes like cell survival, antiviral signaling, cell division, vesicular trafficking, NFkB signaling and autophagy. Optineurin acts as an adaptor which recruits other proteins to their site of action in several signaling pathways. Mutations in optineurin are reported to be involved in two neurodegenerative diseases- glaucoma and amyotrophic lateral sclerosis (ALS). Glaucoma affects retinal ganglionic cells, whereas, in ALS, motor neurons of the cortex, spinal cord and brainstem progressively degenerate. Impairment in the process of autophagy by mutants of optineurin is one of the mechanisms involved in the pathogenesis of both glaucoma and ALS. Autophagy is a process by which cellular wastes, mutant proteins and defective organelles are degraded through lysosomes. The process initiates with the formation of ‘phagophore’- a double membraned, cup-shaped structure, which engulfs the cytoplasmic components or the cargo to be degraded. The ends of the phagophore fuse to form ‘autophogosomes’, which fuse with ‘lysosomes’ to form ‘autolysosomes’. In the autolysosomes, pH-dependent degradation process takes place. LC3 is an autophagosomal membrane protein and plays a crucial role in the process of autophagy. Optineurin is an adaptor protein which recruits the cargo to be degraded to LC3 positive autophagosomes. It has two domains important for its function in autophagy- a cargo recognizing ubiquitin-binding domain (UBD) and an LC3 interacting region (LIR). Thus it acts as an autophagy receptor which recruits the cargo to be degraded to the autophagosomes. Optineurin is also involved in the formation of autophagosomes and the fusion of autophagosomes with lysosomes to form autolysosomes.
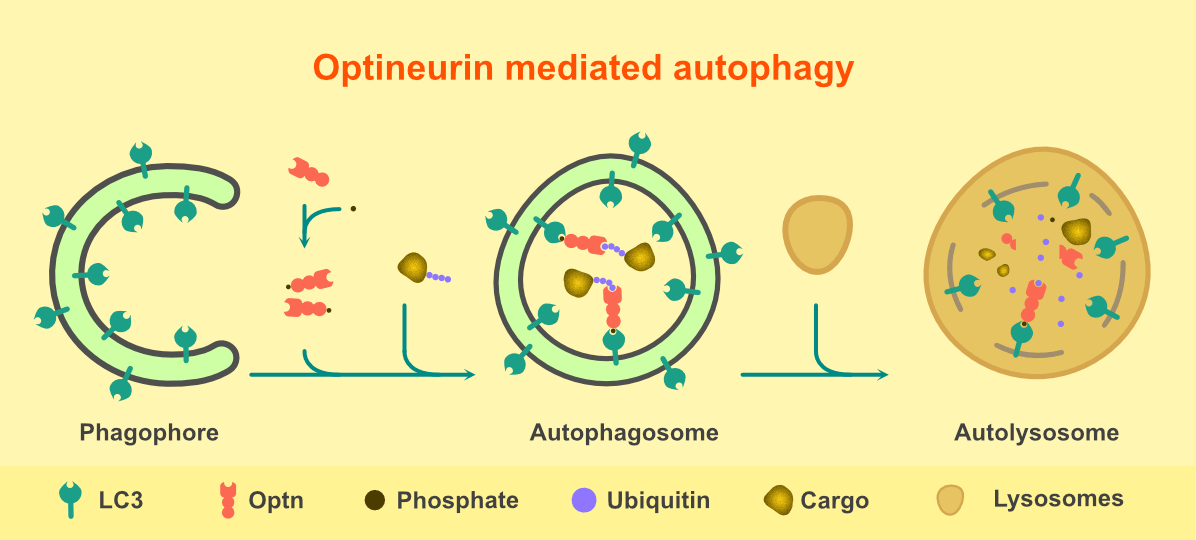 Figure 1. Steps involved in Optineurin mediated autophagy
Figure 1. Steps involved in Optineurin mediated autophagy
To understand the cellular functions of optineurin and its relevance in disease, optineurin (Optn-/-) knockout mice were generated. We observed that the tissues and cells derived from these Optn-/- mice did not have full-length optineurin protein, however, they showed higher expression of a protein form having molecular weight less than optineurin and recognized by optineurin antibody specific for C- terminal region. This form was also down-regulated by optineurin ShRNA. We confirmed that this truncated optineurin protein, which was translated from a splice variant of full length optineurin, lacked N-terminal 157 amino acids of full length optineurin. We named it d157mOptn. d157mOptn is expressed in cells and tissues derived from wild type mice, but its levels are extremely low at normal conditions. We explored the function of d157mOptn in autophagy and observed that, unlike full length optineurin, d157mOptn is defective in autophagic flux and promoting autophagosome and autolysosome formation. This defect in d157mOptn is due to lack of phosphorylation resulting due to impaired interaction with a kinase, TBK1. The N terminal region of full length optineurin, which is absent in d157mOptn, is required for interaction with TBK1. Phosphorylation of optineurin by TBK1 is important for its interaction with the autophagosomal protein, LC3 and thus plays a crucial role in autophagy. Thus d157mOptn is defective in autophagy due to impaired interaction with TBK1 and loss of phosphorylation. Unlike full length optineurin, d157mOptn is compromised in clearing mutant protein aggregates through autophagy. Taken together, d157mOptn is defective in basal and cargo-selective autophagy.
Thus, we have identified and characterized a novel isoform of mouse optineurin which is impaired in autophagy, unlike its full length counterpart. Full length optineurin is known to form functional multimers. We observed that d157mOptn also intercalates in the multimers formed by optineurin. The level of d157mOptn in normal conditions is very low. However, increased level of d157mOptn in the cells can have a counteracting effect on the function of full length optineurin in the process of autophagy by intercalating in the complexes formed by full length optineurin. Thus it is possible that d157mOptn can regulate the autophagic function of full length optineurin and might have some significance in diseased condition.
Primary reference:
[simple-author-box]
