MacConkey agar
Introduction
MacConkey agar (MAC) was developed as the first solid differential media in the 20th century by a bacteriologist, Alfred Theodore MacConkey. While working for the ‘Royal Commission’ on sewage disposal, his role was to inspect drinking water sources for gram negative enteric organisms. These bacteria usually inhabit the gastrointestinal tract of humans and other animals. Their presence is an indicator of fecal contamination and could indicate the presence of potentially pathogenic bacteria.
MacConkey agar is a selective and differential media used to isolate non-fastidious gram negative rods (primarily, the family Enterobacteriaceae and the genus Pseudomonas) and differentiate the bacteria on their ability to ferment lactose.
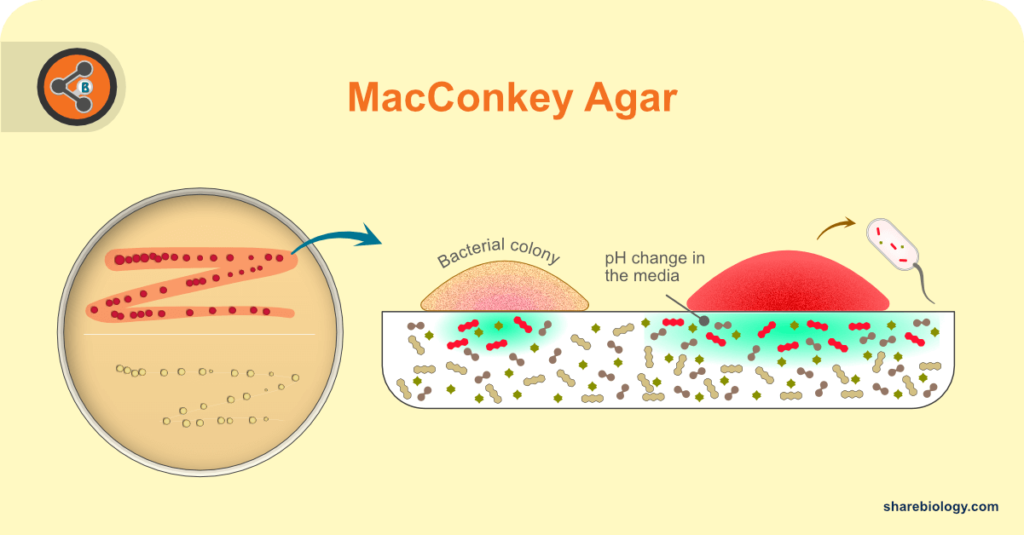
Figure 1. Photograph of MacConkey agar plate with lactose fermenting and lactose non-fermenting bacteria.
Principle
To understand how MacConkey agar works, let’s first see the role of different ingredients.
Agar: Solidifying agent.
NaCl: Osmotic balancer.
Peptone: Nitrogen source, also works as a carbon source for bacteria that can not metabolize sugars.
Bile salts: Inhibit the growth of gram positive bacteria by disrupting the membranes. Gram negative species that live in the colon can resist bile salt’s negative effects.
Crystal violet: Inhibits the growth of gram positive bacteria by inducing DNA lesions.
Neutral red: A pH indicator that acts as a litmus paper. Turns to red/pink when pH drops below 6.8.
Lactose: A sugar that works as a carbon source. Some bacteria consume lactose and produce acids (lactose fermenters). When the acids are released into surrounding media, media pH drops.
The color of colonies in MacConkey agar is based on their ability to change the pH of the media. For more details on pH changes, read EMB Agar.
Strong lactose fermenters: Escherichia coli is a typical example of this group. These bacteria drastically change the pH of the surrounding media to acidic, changing the color of neutral red and precipitating the bile salts. The dye is also taken up by the bacterial cells resulting in the pink colonies, and the precipitation of bile salts results in a ‘pink halo’. A ‘Pink halo’ is a narrow white region at the pink color junction (produced by colonies) and the pH-unaffected area.
Weak lactose fermenters: Serratia and Enterobacter aerogenes are an example of this group. These species change the pH of media to acidic but not as much as the strong fermenters do. The drop in pH is just enough to change the color of the indicator dye. These bacteria also take up the dye, giving a pink appearance to colonies. However, they do not drop the pH of the media to the extent of bile salts precipitation.
Colonies that do not change the pH of media: Salmonella, Proteus species, Yersinia, Pseudomonas aeruginosa, and Shigella are examples of this group. These bacteria do not ferment lactose; hence neutral red does not change to pink. Some of these bacteria can change the pH to alkali due to protein deamination. Increasing the pH of the media may result in a change in the color of media to yellow as neutral red turns yellow at alkaline pH.
Figure 2. Illustration depicting the mechanism of color development in the lactose fermenting bacterial colonies.
Applications
- MacConkey agar is routinely used as a selective media for the isolation of non-fastidious gram negative bacteria from wounds, stool, urine, and blood samples.
- It is used as a differential media and an indicator media (neutral red pH indicator) to distinguish Gram-negative bacteria that can ferment the lactose from those that cannot.
- Isolating and analyzing the count of coliforms and intestinal pathogens using MacConkey agar is helpful in testing the quality of water and dairy products.
Composition
| Ingredient | For 500 ml of media | For 1L of media |
|---|---|---|
| Peptone | 1.5 gm | 3 gm |
| Pancreatic digest of gelatin | 8.5 gm | 17 gm |
| Lactose | 5 gm | 10 gm |
| Bile salts | 0.75 gm | 1.5 gm |
| NaCl | 2.5 gm | 5 gm |
| Crystal violet | 0.0005 gm (0.5 mg) | 0.001 gm (1 mg) |
| Neutral red | 0.015 gm (15 mg) | 0.030 gm (30 mg) |
| Agar | 6.75 gm | 13.5 gm |
Table 1. Composition of MacConkey agar medium.
As per the necessity of the user, the fermentable sugar lactose can be replaced in the medium by other sugars. The ability of gram-negative bacteria to ferment these replacement sugars is analyzed in the same way as lactose fermentation.
pH
The pH of the MacConkey agar medium is about 7.1+/- 0.2. Adjust the pH with 1N NaOH and HCl.
Preparation
- Weigh the ingredients separately with respect to the volume of the media. (Here, we are considering 1L of the media).
- Suspend the ingredients (except agar) in a glass beaker containing about 900mL of distilled water.
- Dissolve the components in the beaker using a magnetic stirrer. (Heat may be applied to dissolve the medium completely).
- Adjust the pH of the medium to the desired value.
- Adjust the broth to a final volume of 1L using distilled water
- Transfer the broth to a conical flask or aliquot into smaller volumes.
- Now add agar accordingly with respect to the volume of the media (i.e., 13.5 gm agar for 1L of the media).
- Close the mouth of the flask with a cotton plug. Seal it further with paper and a rubber band.
- Autoclave for 20 min at 15 psi (1.05kg/cm2) on liquid cycle.
- However, if antibiotics are to be included, their stock solutions should be filter sterilized prior to addition into the media. These antibiotics must be added after the media is cooled to about 45-50°C.
- Mix well and pour into sterile Petri plates or tubes for slants.
Alternatively, commercially available MacConkey agar media powders can be used. Weigh the mixture of content as prescribed by the manufacturer.
Storage
Store the media plates at 4°C until they are utilized.
Points to be noted
Add agar after adjusting the pH of the media. At acidic pH, agar hydrolyzes and results in soft agar plates.
Limitations
- Only presumptive identification is possible by observing colony morphology. However, for the final identification, they have to be subcultured, and confirmation tests should be done.
- Some strains may show reduced growth, or they may fail to grow on this medium.
- Increased levels of CO2 during incubation of MacConkey Agar plates have been reported to reduce the growth and recovery of a number of strains of Gram-negative bacilli.