Note: This article is for information purpose only. Not intended as medical advice.
Introduction
In mid-1960s first human coronavirus has been identified [1]. The presence of spikes gives the appearance of a crown, hence the name coronavirus. There are four main sub-groups of coronaviruses, i.e. alpha, beta, gamma, and delta. Coronaviruses are a syndicate of related RNA viruses which may typically cause illness in humans and other animals. These viruses can affect the respiratory system, exhibits the common cold to more severe conditions. There are many coronavirus outbreaks in history [2].
| Type of coronavirus | Name of coronavirus and their sub-groupings |
|---|---|
| Common human coronaviruses | 229E (alpha coronavirus) |
| NL63 (alpha coronavirus) | |
| OC43 (beta coronavirus) | |
| HKU1 (beta coronavirus) | |
| Other human coronaviruses | MERS-CoV (beta coronavirus that causes Middle East Respiratory Syndrome, or MERS) |
| SARS-CoV (beta coronavirus that causes severe acute respiratory syndrome, or SARS) | |
| SARS-CoV-2 (the novel coronavirus that causes coronavirus disease 2019, or COVID-19) |
COVID 19 (SARS-CoV-2)
In December 2019 new virus and disease were came to light due to outbreak began in Wuhan, China, which is named as a SARS-CoV-2 and disease as COVID 19[3]. The most common symptoms include fever, dry cough and tiredness and in some cases also containing aches and pains, nasal congestion, headache, conjunctivitis, sore throat, diarrhea, loss of taste or smell, rashes on skin or discoloration of fingers or toes[4].
From recent reports, a study on the spike protein suggests that spike protein grab the outer walls (cell membrane) of the host’s cells and then freely enters the host cells. Functionally, the spike contains two parts, grabber and cleavage site. Together, it allows the binding of virus (grabber) to its host and allows the entry (cleavage site) into human cells [6].
Current COVID 19 therapeutics
Till now there no FDA approved drugs available for this disease. On March 3rd and May 1st FDA approved two drugs, i.e. Hydroxy Chloroquine (HCQ) and Remdesivir for the Emergency Use Authorization (EUA).
Hydroxy-Chloroquine (HCQ)
Hydroxychloroquine is used to treat malaria, lupus erythematosus and rheumatoid arthritis. From some multicentred clinical trials and cell-cultured studies, it is evident that chloroquine may potentially display therapeutic efficacy against COVID-19[7]. However, the US Food and Drug Administration (FDA) has cleared emergency use of hydroxychloroquine in adults and adolescents weighing at least 110 pounds (50 kilograms) who are hospitalized with COVID-19.
Treatment
HCQ may inhibit SARS-CoV-2 in-vitro with EC50=0.72%µM, which is more potent than chloroquine (EC50=5.47%µM)[8]. Azithromycin has been exhibited to be active in-vitro against Zika and Ebola viruses and to prevent severe respiratory tract infections when administrated to patients suffering from viral infection. Instead of single drug HCQ, a combination of HCQ with Azithromycin has shown excellent control over the decease progression. A recent clinical study reported that at day6 post-inclusion, 100% of patients treated with hydroxychloroquine and azithromycin combination were virologically cured comparing with 57.1% in patients treated with HCQ only, and 12.5% in the control group[8]. Administration of HCQ by the oral route is easy and goes into the systemic circulation.
Side effects
HCQ have severe side effects, including improved pneumonia. The other side effects include abnormal heart rhythms, dangerously rapid heart rate, skin rashes, fever, swollen glands, muscle aches, severe weakness, unusual bruising, or yellowing of skin or eyes [9].
REMDESIVIR
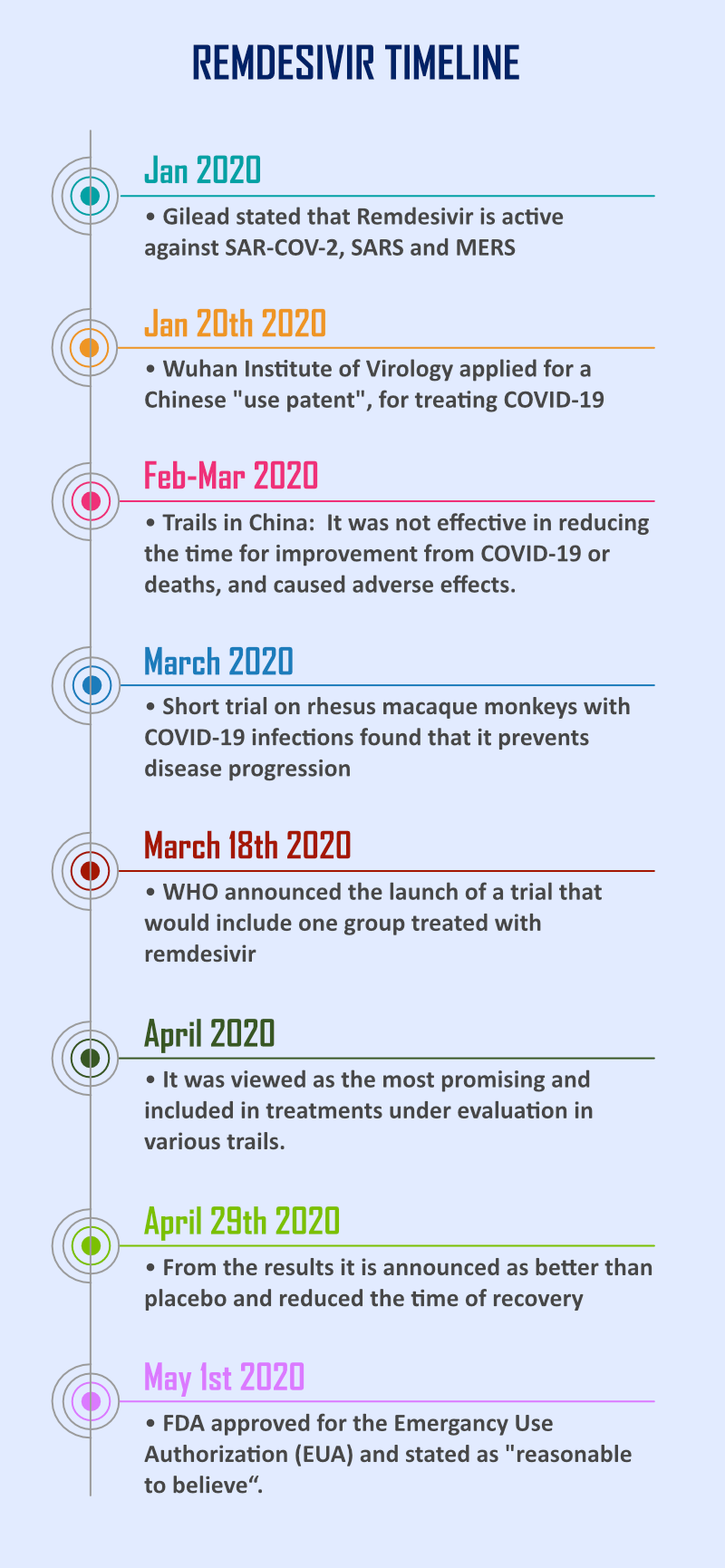
Remdesivir is an antiviral medication developed for a broad-spectrum viral related disease [10]. It was initially made to treat hepatitis C and tested against Ebola virus and Marburg virus diseases but was not effective for these infections. This drug candidate was also gone through clinical studies for other RNA viruses such as SARS coronavirus and MERS coronavirus but did not get approved for any medication. In 2015 from the clinical data on Rhesus monkeys depicts that it can able block Ebola virus effectively. Different clinical studies conducted from different countries produce contradictory data in treating COVID‑19 [11].
Treatment
Remdesivir mechanism of action includes decrease in viral RNA production by interfering with the action of viral RNA-dependent RNA polymerase (an enzyme makes copies of viral genetic material) and evades proofreading by viral exoribonuclease [12]. In the case of MERS-CoV, SARS-CoV-1, and SARS-CoV-2, depends on RNA-Dependent RNA Polymerase associated with the RNA synthesis can be arrested by Remdesivir active metabolite. Hence this drug is categorized as a delayed chain terminator [14]. Early clinical data from a controlled trial carried out by the U.S. National Institutes of Health (USNIH), shows that remdesivir is effective in reducing the recovery time from 15 to 11 days in people seriously ill with COVID‑19 [15].
Side effects
Every drug has its demerits, in this case, increased liver enzyme levels that may indicate possible liver damage. Some Reports confirmed similar increases in liver enzymes in three U.S. COVID-19 patients, Nausea, Vomiting, respiratory failure, blood biomarkers of organ impairment, low count of platelets, gastrointestinal distress, elevated transaminase levels in the blood [16].
[simple-author-box]
